Lysosomes are membrane-enclosed organelles that contain an array of enzymes capable of breaking down all types of biological polymers-proteins, nucleic acids, carbohydrates, and lipids. They frequently fuse with a number of cellular organelles such as endosomes, autophagosomes, phagosomes and the plasma membrane, playing important roles in endocytic, phagocytic, autophagic, and exocytotic membrane-trafficking pathways. Emerging evidence suggests that lysosomal membrane proteins, including ion channels and transporters, are essential for lysosome functions. Primary interests of my lab are lysosomal ion channels and transporters in lysosome physiology, calcium signalling, membrane trafficking, autophagy, and their pathological implications. Investigation of lysosomal channels and transporters will facilitate our understanding of the pathogenic mechanisms of many human diseases, such as lysosomal storage diseases, neurodegenerative diseases, cancers, myopathies, and bacterial and virus infection.
Techniques used in our laboratory include whole-cell patch-clamp electrophysiology, lysosome patch-clamp electrophysiology, calcium imaging, molecular/cellular biology, biochemistry, confocal microscopy, and mouse genetics.
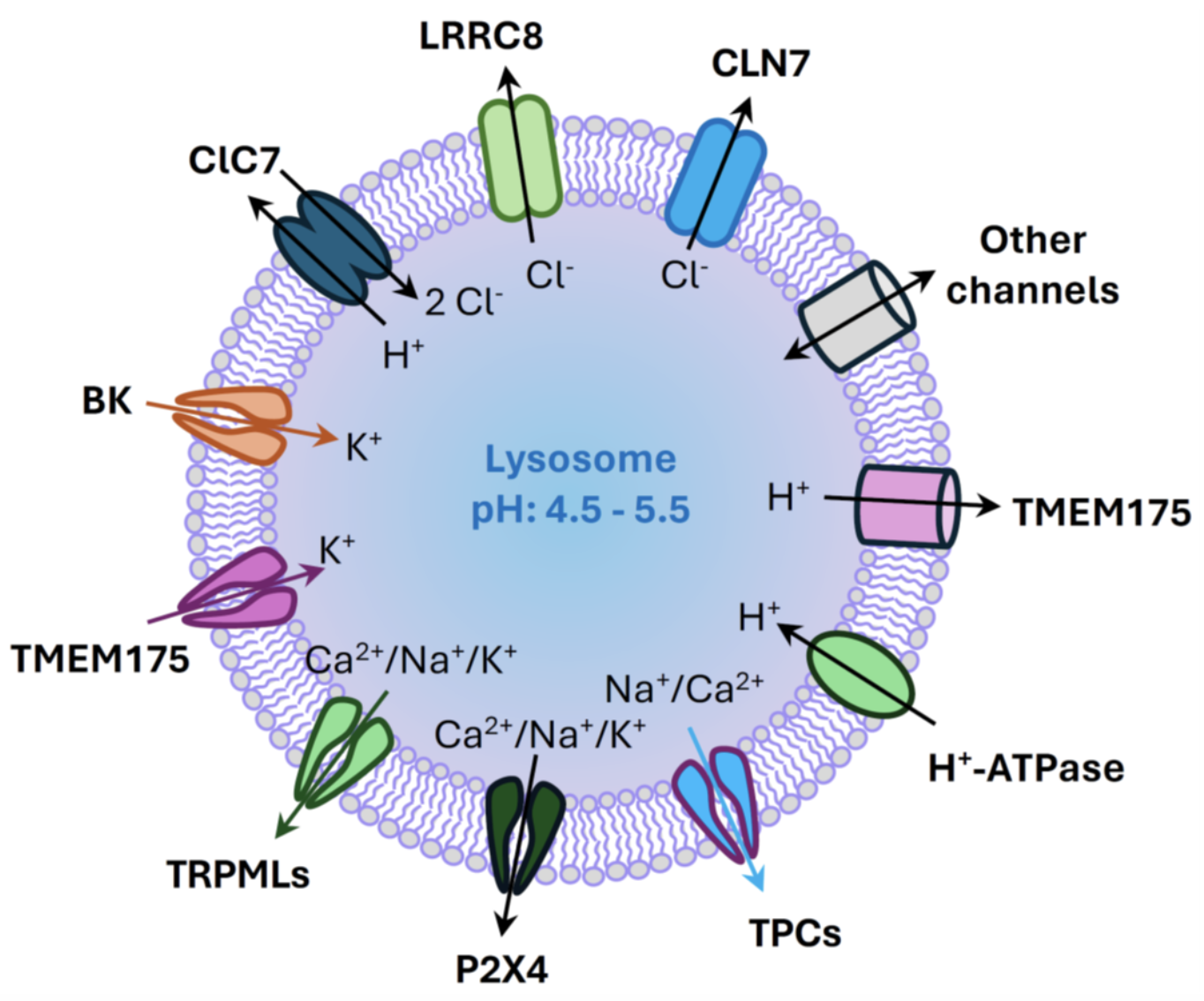
Schematic diagram illustrating ion transport pathways in the lysosome
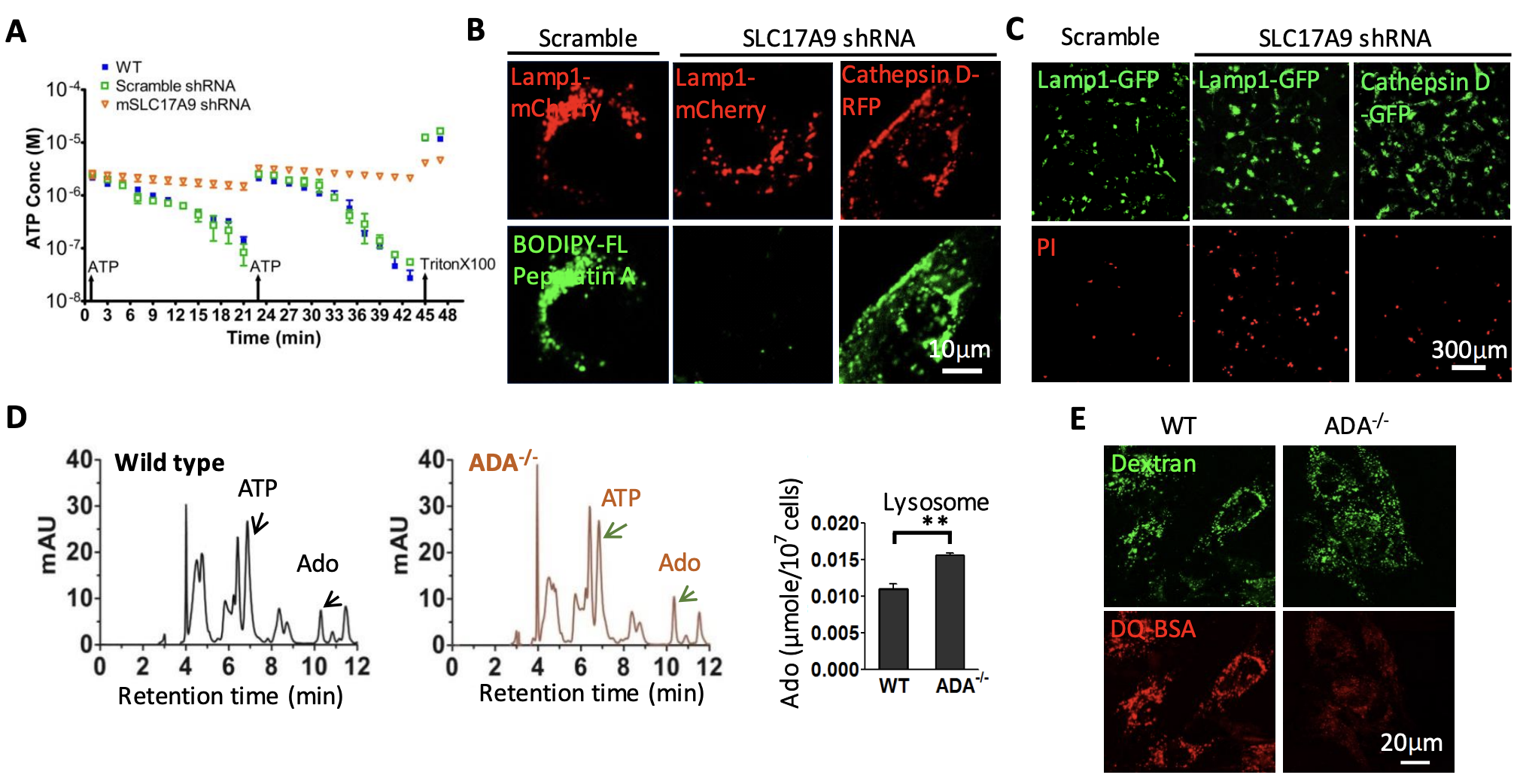
(A-C) SLC17A9 transport cytosolic ATP into lysosomes to activate cathepsin D, which is essential for cell survival (J Biol Chem. 2014, 289:23189- 99; J Physiol. 2016, 594:4253-66; Cells. 2022, 11:887). (D, E) Lysosomal ATP can also be degraded to adenosine, which accumulation leads to TRPML1 inhibition and subsequent lysosomal dysfunction and cell death. This has been involved in in severe combined immunodeficiency diseases (J Biol Chem. 2017, 292:3445-3455). ADA: Adenosine deaminase; SLC17A9: Solute carrier family 17 member 9. BODIPY-FL Pepstatin A: Cathepsin D indicator. PI: Propidium iodide.

(A) SLC17A9 transports cytosolic ATP into lysosomes where ATP activates P2X4 when luminal pH is high (J Biol Chem. 2014, 289:23189- 99). (B) Activated P2X4 stimulates calmodulin to promote lysosomal membrane fusion (J Biol Chem. 2014, 289:17658-67; J Cell Biol. 2015, 209:879-94). (C) On the other hand, TRPML1 is activated by PI3,5P2 when luminal pH is low. Activated TRPML1 stimulates calmodulin-mTOR complex to faciliate lysosome fission (J Biol Chem. 2017, 292:8424-8435; Eur J Cell Biol. 2019, 98:116-123).ML-SA1: TRPML activator.
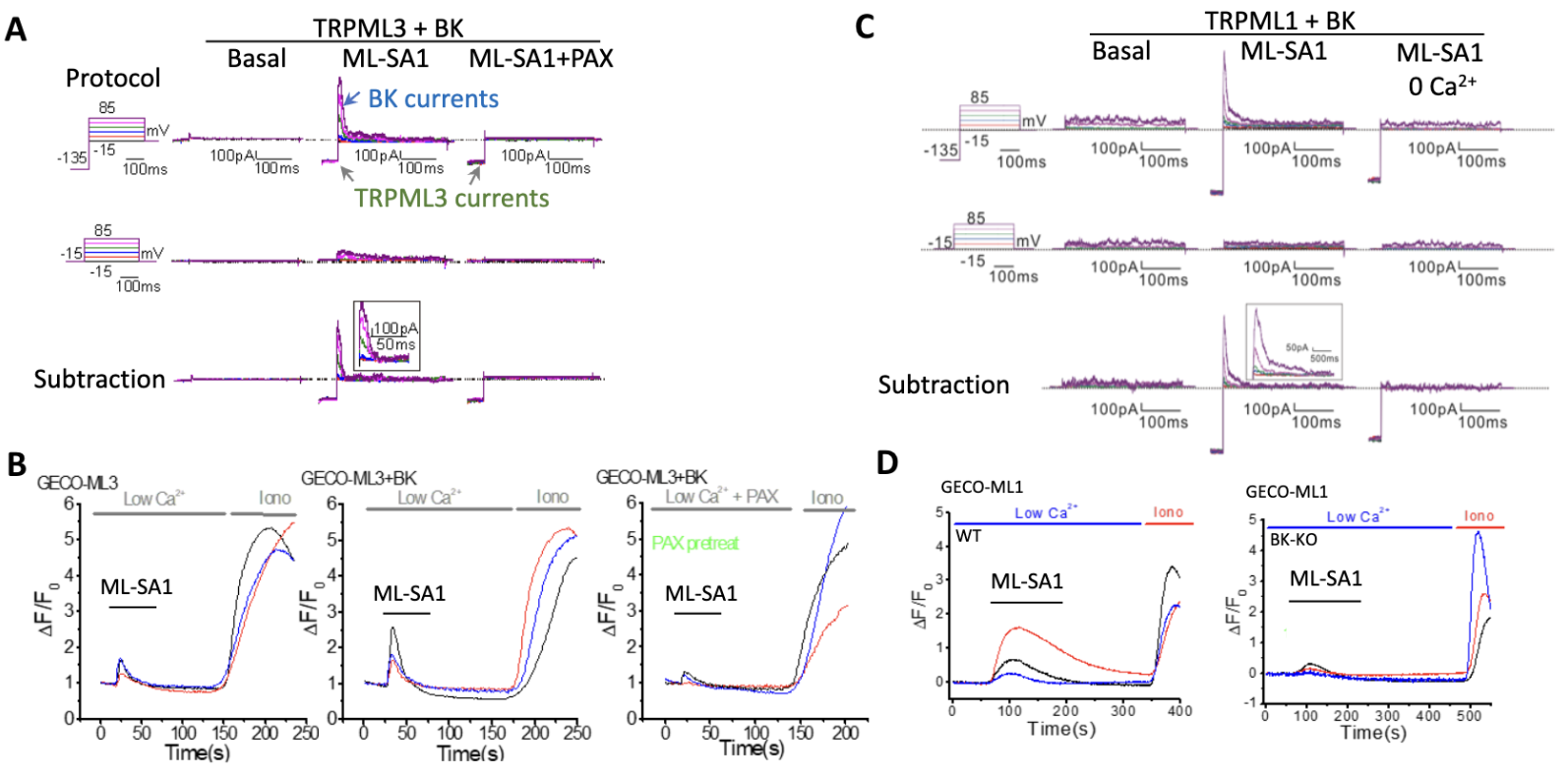
Both TRPML3 (A, B) and TRPML1 (C, D) form a positive feedback loop with BK. Calcium release from endolysosomes via TRPML1 and TRPML3 activates BK, and BK in turn facilitates TRPML1 and TRPML3 (Dev Cell. 2015, 33:427-41; PNAS. 2023, 120:e2215777120). ML-SA1: TRPML activator; PAX: BK inhibitor

TRPML1 and TRPML3 are two calcium release channels residing in the endolysosomal membrane. They are activated by phosphatidylinositol (PtdIns)-3-phosphate (PtdIns3P) and/or PtdIns(3,5)P2. Their activities are also regulated by luminal pH, with low pH enhancing that of TRPML1 and high pH increasing that of TRPML3. TRPML1 and TRPML3 are activated by a reduction in mTORC1 activity; their activation in turn regulates mTORC1 activity to facilitate macroautophagic/autophagic flux. On the one hand, TRPML3 appears to be recruited to phagophores where it is activated by PtdIns3P and high pH to inhibit mTORC1 activity using a positive feedback mechanism, thereby increasing autophagy induction (A-C) (PNAS. 2023, 120:e2215777120). On the other hand, TRPML1 is activated by PtdIns(3,5)P2 and low pH in (auto)lysosomes to increase mTORC1 activity using a negative feedback mechanism, promoting autophagic lysosome reformation (D-F) (Autophagy, 2018, 14:38-52). The cell uses the two feedback mechanisms to ensure efficient autophagic flux to survive adverse conditions such as nutrient deprivation and bacterial infection. SAR405: VPS34 inhibitor; YM201636: PIKfyve inhibitor.
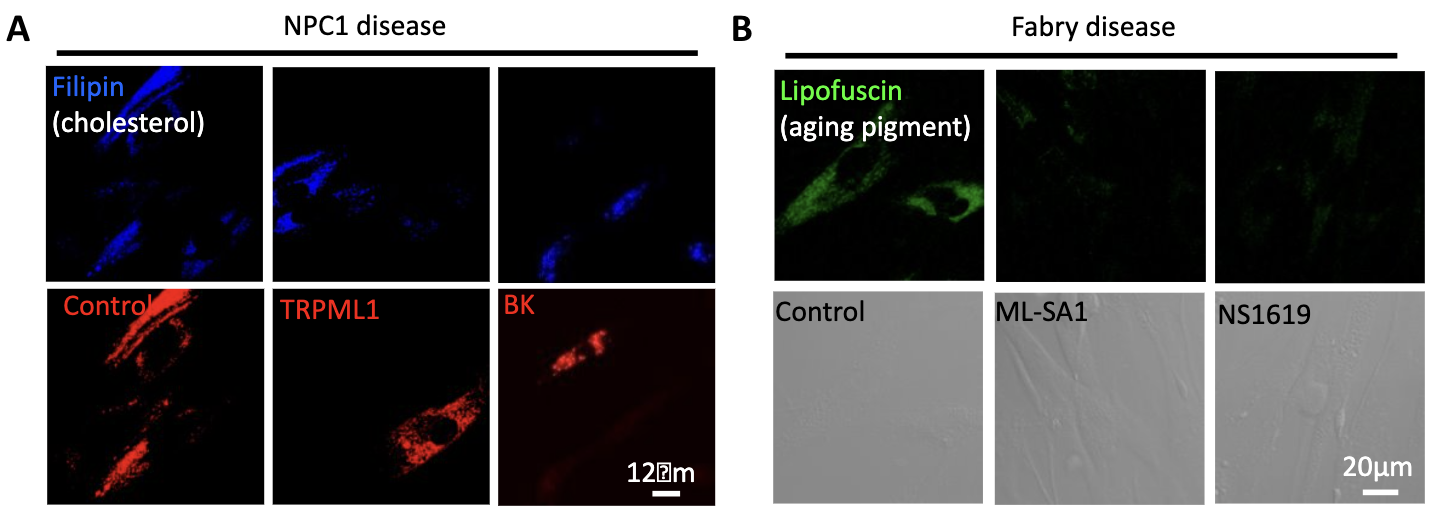
TRPML1 and BK facilitate the clearance of lysosomal storage by promoting calcium-dependent lysosomal exocytosis,suggesting that upregulating TRPML1-BK may potentially treat certain lysosomal storage diseases including mild cases of Mucolipidosis type IV (ML4), Niemann-Pick type A (NPA) and Fabry diseases (Dev Cell. 2015, 33:427-41. Sci Rep. 2016, 6:33684).
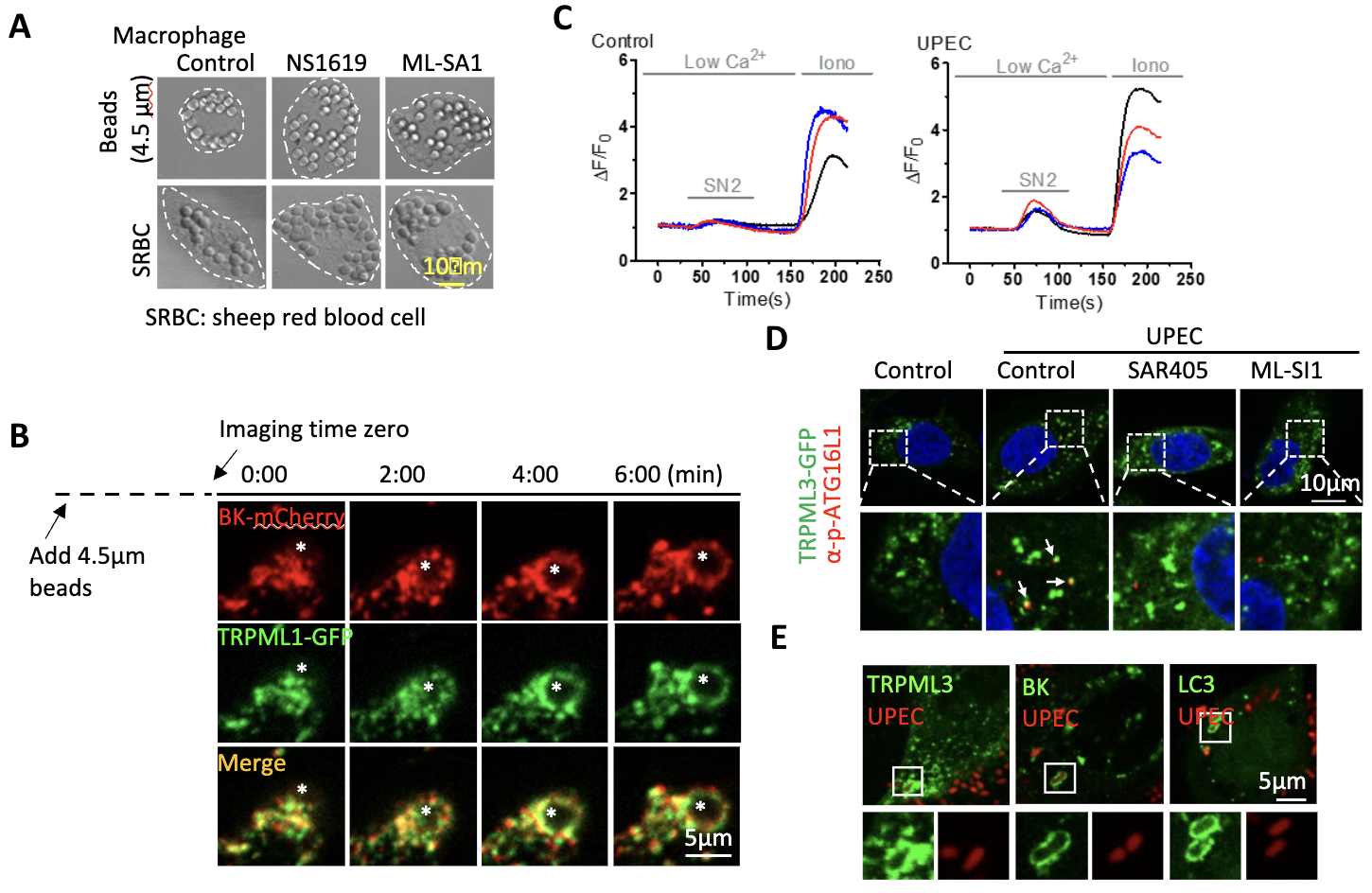
(A, B) TRPML1/BK promote the phagocytosis of large particles (Sci Rep. 2020, 10:1038). (C-E) Uropathogenic E. coli (UPEC) activates TRPML3/BK complex to promote autophagy induction and bacterial clearance (PNAS. 2023, 120:e2215777120). NS1619: BK agonist; ML-SA1: TRPML agonist; SN2: TRPML3 activator; ML-SI1: TRPML inhibitor; SAR405: VPS34 inhibitor.

Genetic downregulation and pharmacological inhibition of TRPML1 suppress triple negative breast cancer growth by inhibiting mTOR (A) and metastasis by inhibiting lysosomal ATP release (B) (Cell Calcium. 2019, 79:80-88.).
By regulating endocytic, phagocytic, autophagic, and exocytotic membrane-trafficking pathways, lysosomal ion channels may be implicated in neurodegeneration using both cell autonomous and non-cell autonomous mechanisms (A) andmyogenesis during early development and after muscle injury (B). We hope to develop new strategies targeting on lysosomal ion channels to suppress the progression of some neurodegenerative diseases and myopathies, as well as other human diseases.